
The H5N1 vaccine UKHSA purchased from CSL/Sequirus LTD
Maybe
The UKHSA purchased over 5 million doses of an Influenza H5N1 pandemic vaccine from CSL/Sequirus LTD. The UKHSA is highly secretive about its actions, so we have had to work out for ourselves that the stuff they bought or optioned is most likely AUDENZ (In Europe, AUDENZ is called Incellipan):
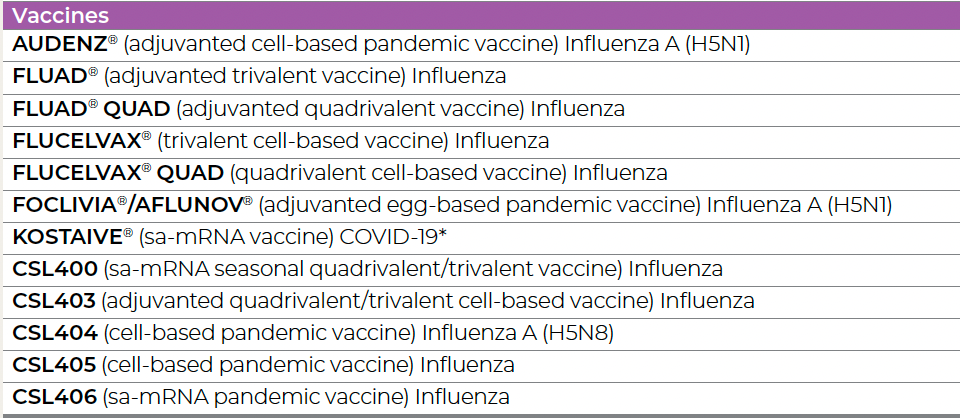
This is taken from page 23 of CSL’s Annual Report to Investors 2024. You will be interested to know that the FDA has not licensed any of the CSL4 series influenza vaccines so far.
So, let’s assume the British taxpayer has invested in Audenz. The FDA licensed the vaccine on 24 April 2024 “for use in persons 6 months of age and older at increased risk of exposure to the influenza A virus H5N1 subtype contained in the vaccine.”
Mark the words, folks.
As you know, we look at regulatory data, not commercial publications in biomedical journals, so we went to the US FDA documentation. It’s worth looking at the accelerated approval letters, which show the progressive development of the marketing plan over several years with expanding age groups. But let’s stick to the final version.
The manufacturers ran four trials before the final phase 3 trial, V89_18, which provided sufficient data for final registration.
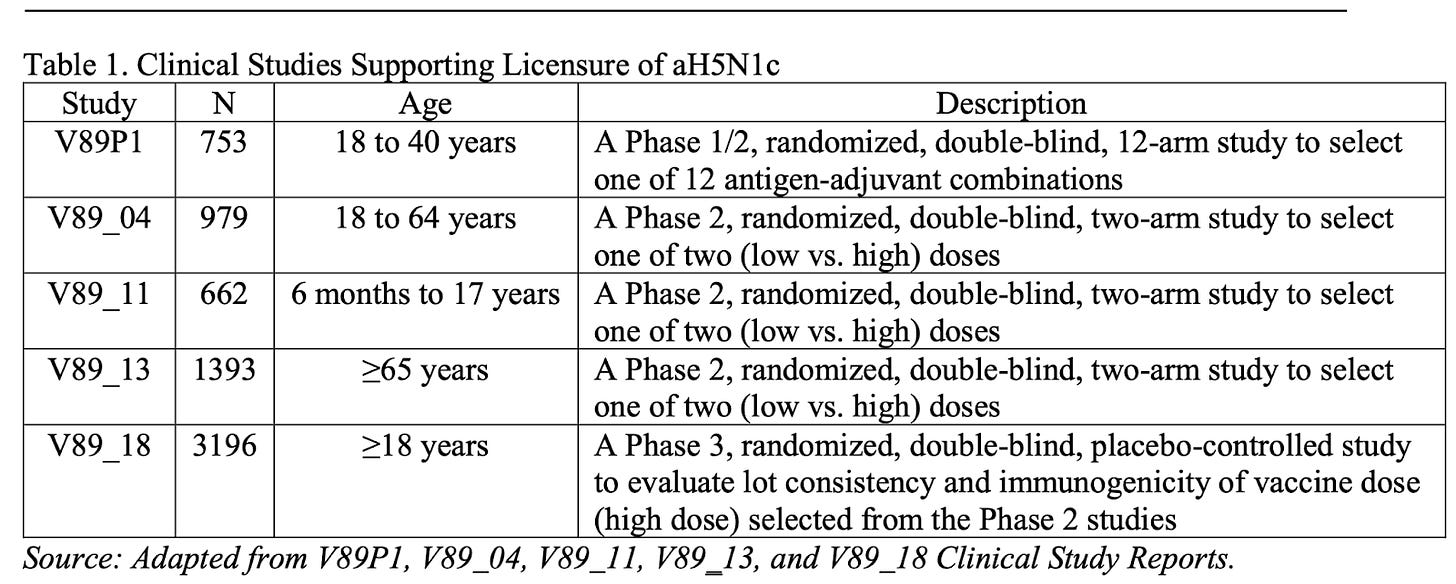
When looking at the table, remember that the outcomes were harms and antibody responses, as there was no H5N1 around in humans to assess field protection.
The FDA statisticians were all sweetness and light, not noting any problems with the trials and their 6983 participants.
However, In trial 04, four deaths occurred in the 468 higher antigenic concentration arm recipients (7.5 µg H5 hemagglutinin antigen + 0.25 mL MF59 adjuvant). Still, these deaths were not considered vaccine-related, but one abortion was possibly related to vaccine exposure. Ditto for study 18, the phase 3 trial in which 11 deaths in the active treatment and 1 in placebo were not considered vaccine-related. A possible dose effect was not discussed, and there is no information on the cause of these four deaths as we haven’t yet found the clinical study report. We’ll keep looking.

So, there is nothing to see here, then. Move along.
When we got to the integrated summary of safety in Table 24 (page 37), we found this:
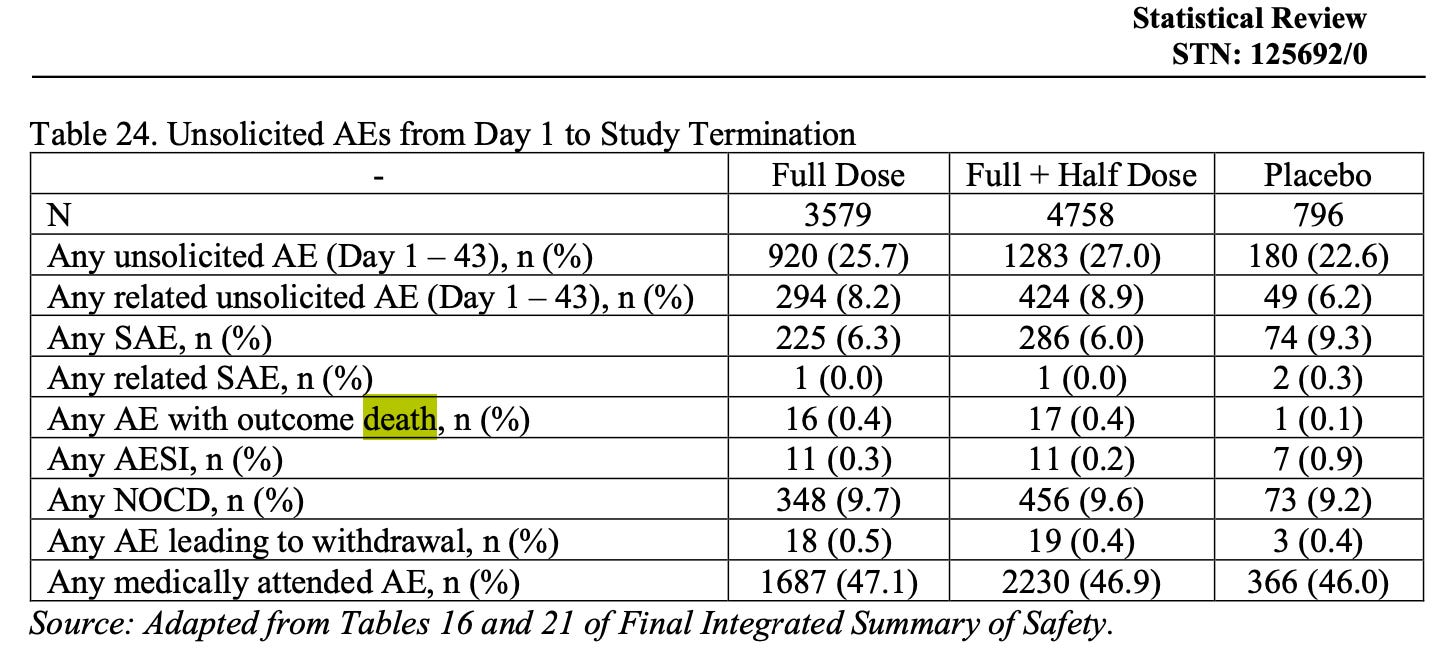

This conclusion warmed the cockles of our frozen hearts, but we found it strange that in a randomised context in which comparability between arms should be very high, 17 vs 1 participants died in the intervention and placebo arms, respectively, none attributable to the intervention, especially since there appears to be a dose-response with the higher dose in trial 04. The relative risk is nearly three-fold = 2.84 (95% CI, 0.38 to 21.3), although the small numbers in the study mean it does not have statistical significance yet (p = 0.31).
Moving on to the EMA, the Risk Management Plan written by Sequirus and approved by EMA is interesting on page 21. It mentions all the rare neurological harms observed in the 2009 Pandemrix, plus vasculitis.
The industry knows that immunising six thousand selected people is one thing; immunising five million is entirely different. That is why we need to understand whether the immunity granted to all other emergency vaccines applies to this vaccine, too.
Please also note that the vaccine has not been tested in pregnant women.

And before we forget, influenza antibody responses are a poor correlate of protection. Governments have been promoting influenza vaccines for decades to avoid winter crises. Have you noticed any difference?
This post was written by two old geezers who do not keep sick chickens under their beds, do not like secrecy, and know when they observe a signal.
This article (The H5N1 vaccine UKHSA purchased from CSL/Sequirus LTD) was created and published by Trust the Evidence and is republished here under “Fair Use”

••••
The Liberty Beacon Project is now expanding at a near exponential rate, and for this we are grateful and excited! But we must also be practical. For 7 years we have not asked for any donations, and have built this project with our own funds as we grew. We are now experiencing ever increasing growing pains due to the large number of websites and projects we represent. So we have just installed donation buttons on our websites and ask that you consider this when you visit them. Nothing is too small. We thank you for all your support and your considerations … (TLB)
••••
Comment Policy: As a privately owned web site, we reserve the right to remove comments that contain spam, advertising, vulgarity, threats of violence, racism, or personal/abusive attacks on other users. This also applies to trolling, the use of more than one alias, or just intentional mischief. Enforcement of this policy is at the discretion of this websites administrators. Repeat offenders may be blocked or permanently banned without prior warning.
••••
Disclaimer: TLB websites contain copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available to our readers under the provisions of “fair use” in an effort to advance a better understanding of political, health, economic and social issues. The material on this site is distributed without profit to those who have expressed a prior interest in receiving it for research and educational purposes. If you wish to use copyrighted material for purposes other than “fair use” you must request permission from the copyright owner.
••••
Disclaimer: The information and opinions shared are for informational purposes only including, but not limited to, text, graphics, images and other material are not intended as medical advice or instruction. Nothing mentioned is intended to be a substitute for professional medical advice, diagnosis or treatment.
Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of The Liberty Beacon Project.






Leave a Reply