CHMP recommends authorization despite grave safety concerns; final regulatory approval now rests with the European Commission
NICOLAS HULSCHER, MPH
On December 12, 2024, the Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion recommending marketing authorization for Kostaive, a self-replicating (replicon) mRNA injection developed by Arcturus Therapeutics. The final decision for regulatory approval now rests with the European Commission:
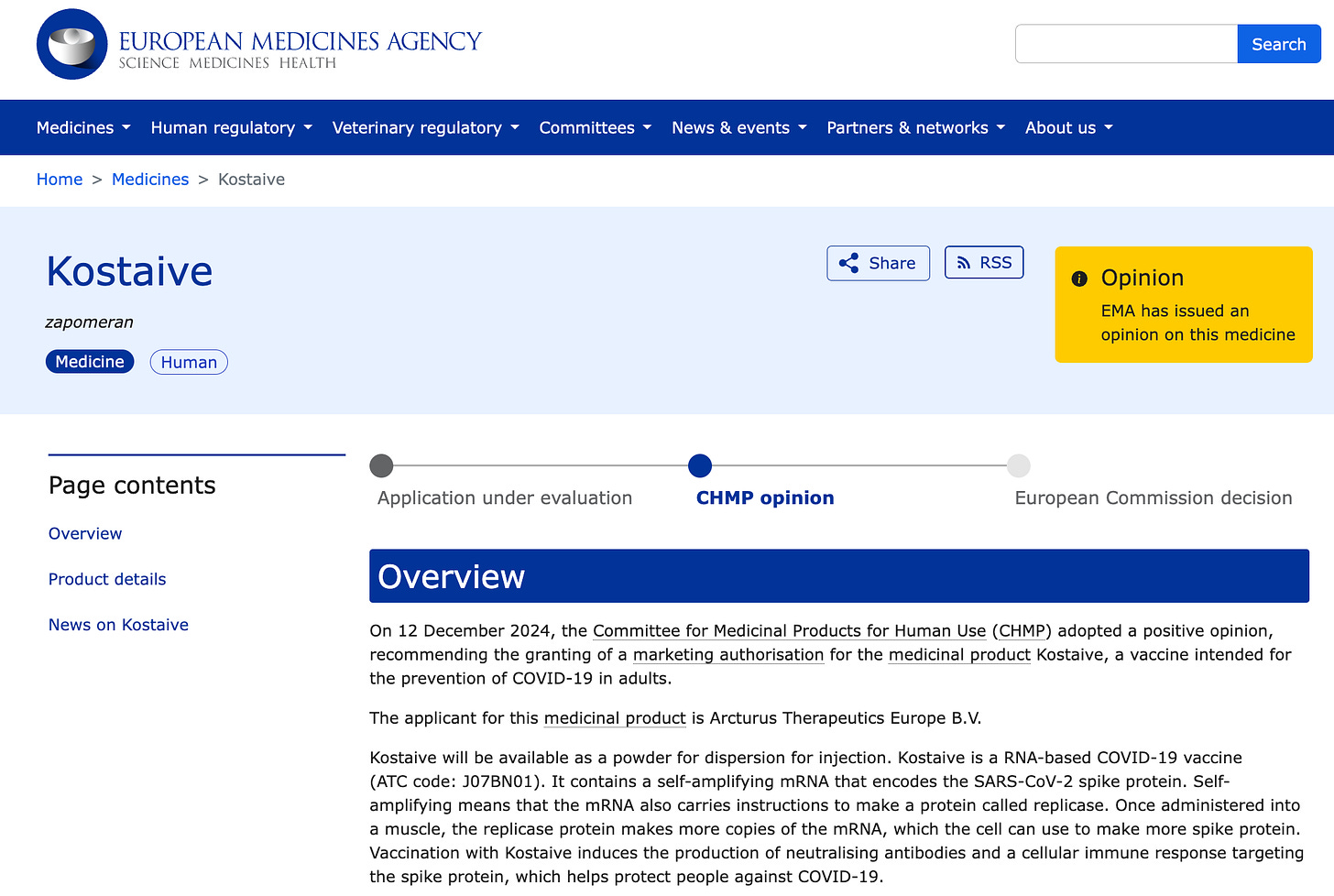
Japan had already approved these injections last year. In November 2023, Japan’s Ministry of Health, Labor and Welfare (MHLW) fully approved CSL and Arcturus Therapeutics’ replicon shot, Kostaive ARCT-154. Despite enormous safety concerns, Japan’s MHLW approved the updated booster shot in September 2024 to target the JN.1 lineage of Omicron subvariants.
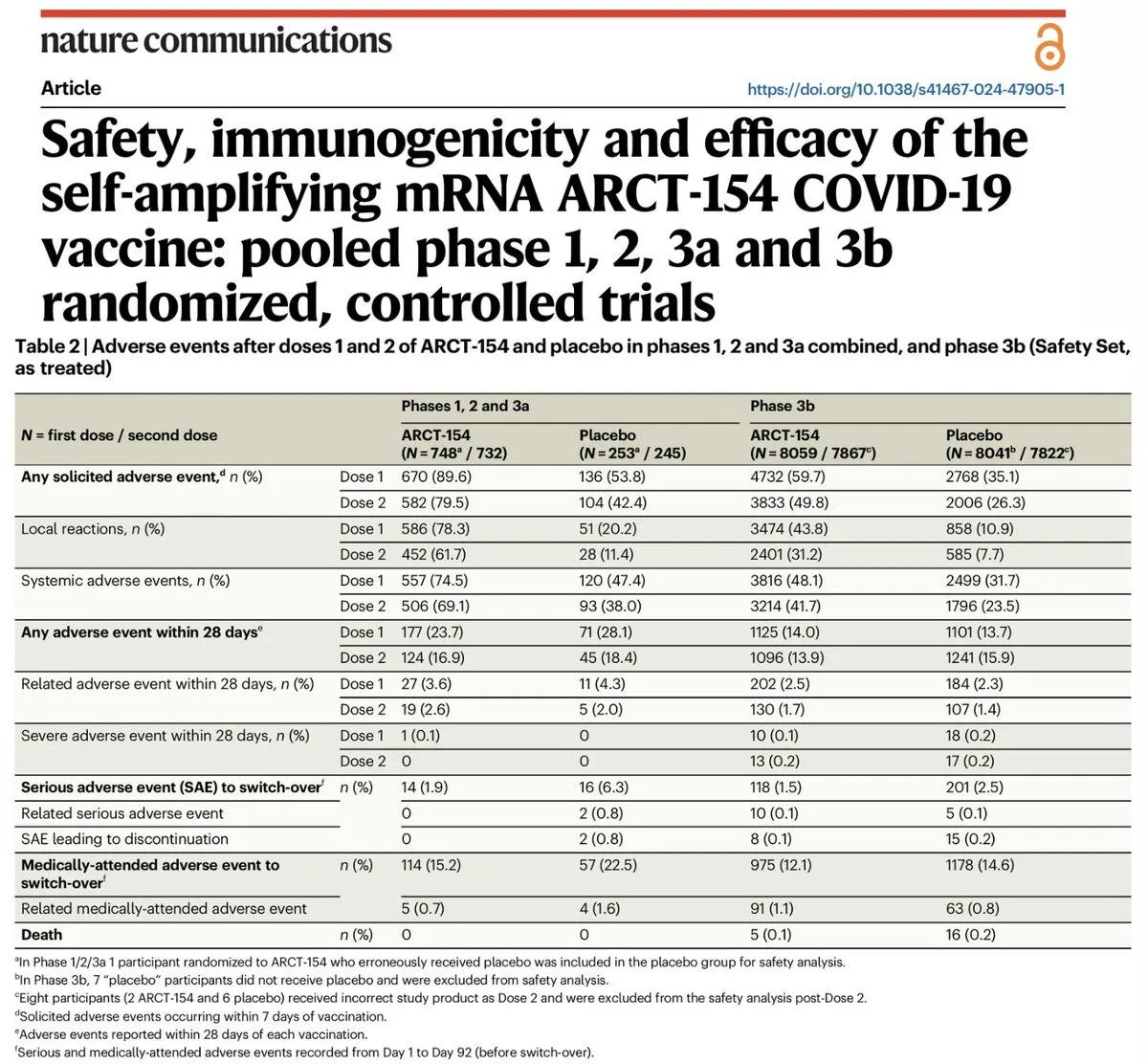
During the clinical trials for Kostaive, five deaths were reported among participants in the phase 3b study. Across study phases 1, 2, and 3a combined, 90% of injected participants experienced adverse events, with 74.5% reporting systemic reactions and 15.2% requiring medical attention after the first dose. Notably, many of the study authors are full-time employees of Arcturus Therapeutics, raising concerns about bias in their conclusions.
As I outlined last month, the Biopharmaceutical Complex’s self-amplifying mRNA assault has already begun with at least 33 candidates in development:
“It’s become abundantly clear that the pharmaceutical industry and captured regulatory agencies have zero regard for the massive safety concerns of undefined synthetic mRNA replication resulting in uncontrolled toxic antigen production. These experimental injections must not receive further regulatory approval for humans or animals if we are to prevent another public health disaster. All self-amplifying mRNA injections currently available for humans and animals should be immediately withdrawn until comprehensive, long-term safety studies are conducted.”
The European Commission must make the right decision and REJECT authorization for an experimental injection with a 90% adverse event rate and non existent long-term safety data.
Nicolas Hulscher, MPH
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
This article (COVID-19 Self-Amplifying mRNA Injection Nears European Approval) was created and published by Courageous Discourse and is republished here under “Fair Use”

••••
The Liberty Beacon Project is now expanding at a near exponential rate, and for this we are grateful and excited! But we must also be practical. For 7 years we have not asked for any donations, and have built this project with our own funds as we grew. We are now experiencing ever increasing growing pains due to the large number of websites and projects we represent. So we have just installed donation buttons on our websites and ask that you consider this when you visit them. Nothing is too small. We thank you for all your support and your considerations … (TLB)
••••
Comment Policy: As a privately owned web site, we reserve the right to remove comments that contain spam, advertising, vulgarity, threats of violence, racism, or personal/abusive attacks on other users. This also applies to trolling, the use of more than one alias, or just intentional mischief. Enforcement of this policy is at the discretion of this websites administrators. Repeat offenders may be blocked or permanently banned without prior warning.
••••
Disclaimer: TLB websites contain copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available to our readers under the provisions of “fair use” in an effort to advance a better understanding of political, health, economic and social issues. The material on this site is distributed without profit to those who have expressed a prior interest in receiving it for research and educational purposes. If you wish to use copyrighted material for purposes other than “fair use” you must request permission from the copyright owner.
••••
Disclaimer: The information and opinions shared are for informational purposes only including, but not limited to, text, graphics, images and other material are not intended as medical advice or instruction. Nothing mentioned is intended to be a substitute for professional medical advice, diagnosis or treatment.
Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of The Liberty Beacon Project.







Leave a Reply